By David Murphy
Air pollution and its insidious effect on our environment remain a leading cause of health issues worldwide. Long-term and short-term exposure to air pollution’s detrimental effects can cause several illnesses, including pneumonia, heart disease, lung cancer and chronic respiratory diseases. According to the World Health Organization, air pollution (both ambient and indoor) accounts for an estimated seven million deaths worldwide each year.
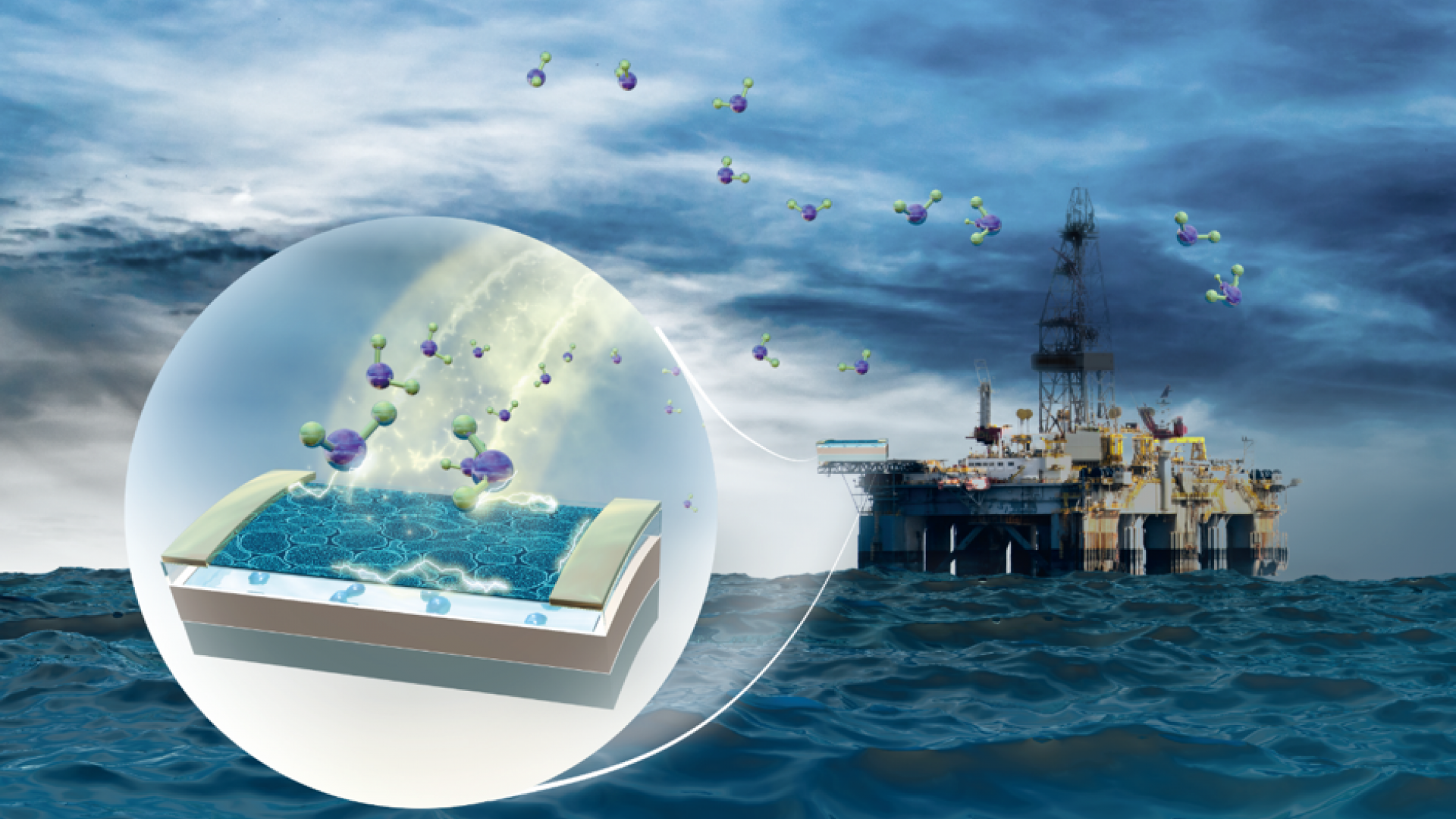
Air pollution comes in many guises and enters our planet’s atmosphere in a host of different ways, including gas emissions from the burning of fossil fuels and chemicals. Exposure to the colorless and foul-smelling gas hydrogen sulfide (H2S) can cause eye irritation, fatigue, neurological damage and cardiovascular issues. Although there are many techniques and devices to detect H2S, they suffer from severe drawbacks, such as larger size, low sensitivity, and high cost.
An interdisciplinary team of researchers from the KAUST Sensors Lab is seeking to address the drawbacks mentioned above by creating a novel p-type organic field-effect transistor (OFET) platform that detects H2S gas selectively. The team’s work has culminated in the recently published paper highlighting the first-time use of hydrazones, a class of organic compounds with the structure R ₁R ₂C=NNH₂, in OFET sensors.
The paper titled “A highly selective electron affinity facilitated H2S sensor: the marriage of tris(keto-hydrazone) and an organic field-effect transistor,” was featured in the high-impact journal for material science and engineering, Materials Horizons. The paper’s authors believe their research can pave the way for the realization of advanced analyte sensing in a multitude of futuristic gas-sensing applications.
“The work presented here is an outcome of multidisciplinary research with far-reaching implications for the broader scientific community and society,” Dr. Sandeep Surya, paper co-author and postdoctoral fellow in the KAUST Sensors Lab, explained.
“This research results from a collaborative effort between different groups working in material science, polymer science, sensors, and electronics. We created a sophisticated OFET device that can sense H2S at very low concentrations. Not just this, the sensor is highly specific to H2S, has a long shelf life, and remains sensitive under diverse conditions, including high humidity levels.”
A novel design for the future of sensors
For their innovative design, the scientists worked with conjugated polymers (CPs), large carbon-based chemical structures that conduct electricity. This ability to conduct electricity can give CPs interesting and useful properties.
The scientists developed a heterostructure OFET device consisting of a CP (PDVT-10) material and a functional organic material called tris(keto-hydrazone), which forms electron interactions with H2S. The device also demonstrates an unprecedented sensitivity (525% ppm−1) and excellent ambient stability (∼5% current degradation after 150 days) compared to traditional sensors.
“Being electron-rich, the receptor compound tris(keto-hydrazone) acts as an electron source and is placed on top of the OFET stack to interact with gas molecules. It is also mildly basic, which allows electronic interaction to occur with an acidic system such as H2S,” Saravanan Yuvaraja, paper first author and Ph.D. student in the KAUST Sensors Lab, explained.
“Importantly, the completed chemical sensor is flat and has the form of a square with a side length of only five millimeters. Thus, it can fit comfortably on a fingertip which makes it easily transportable and readily insertable into restricted spaces. It can also be integrated into larger and more complex circuits, meaning that it could alert computer systems (and the systems’ human users) of the presence of H2S in the air.
“It is minimal in terms of form factor, and the capability to measure surface potential changes gives the device an edge over other conventional sensors. Thus, making it a useful sensor, which is indeed the need of the hour.
“The devices also predict that their sensor system may be modifiable to create sensors for other gases. With just a tweak in the chemical structure and the sensor’s electrophysical properties, one can achieve different sensor applications,” Yuvaraja added.
Devising the latest crop of multi-channel sensors
The current device can underpin new strategies to devise other applications like sensing of biological analytes and ionizing radiation, polymer actuators, photo-switching (smart windows) and drug delivery.
“Eventually, with these hydrazones, it is possible to realize an ingestible device that can sense and neutralize biological entities. It is an added value to the sensor device that these novel materials bring in,” Professor Khaled N. Salama, KAUST CEMSE Division Associate Dean and head of the KAUST Sensors Lab, noted.
The Sensors Lab is also currently working towards developing self-powered gas sensor nodes to detect nitrogen dioxide gas using thin-film transistors made from indium gallium zinc oxide. These self-powered devices can be made into disruptive products with the addition of variants of hydrazone materials.
“Our other works include multi-channel sensors for the detection of cardiac biomarkers. Here, we use different DNA aptamers and antibody coatings specific to cardiac markers like myoglobin Troponin-T, Troponin-I, and fatty-acid-binding proteins.
“We are excited to see our current research featured in such a high-impact journal, especially one that records record performances achieved with novel materials. The Sensors team believes that hydrazones have a long way to go, and we would like to explore other potential applications of these materials in diverse sensing applications. And hopefully, we will get to publish the new findings again in Materials Horizons,” Prof. Salama concluded.