© 2020 KAUST
A simple method developed at KAUST uses laser beams to create graphene electrodes that have better performance than those produced through older methods.
Electrodes consisting of graphene, an atypical form of carbon, may transform the way electroactive substances are detected and measured in numerous fields ranging from food safety and clinical diagnosis to environmental monitoring.
Graphene comprises multiple ultrathin and highly ordered sheets of interconnected honeycomb-shaped rings of carbon atoms. This multilayered architecture provides the material with exceptional electronic properties, especially electrical conductivity and electrocatalytic activity, as well as physical features that are useful for making electrochemical sensors.
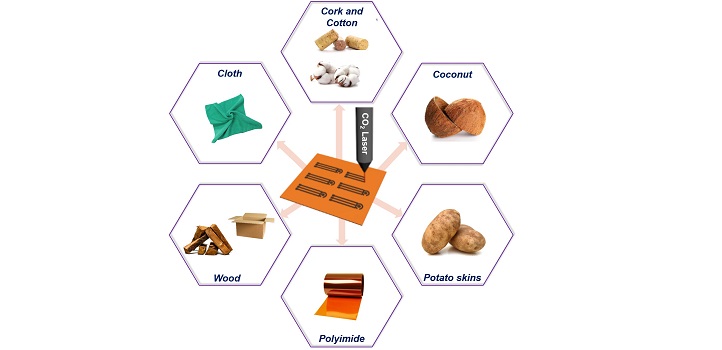
Typically, graphene electrodes are produced by peeling off individual sheets from graphite or depositing a reactive gaseous mixture of precursors onto a substrate. However, these approaches involve time-consuming, multistep synthesis and isolation processes; plus, they struggle to control stacking and oxidation of the sheets.
To improve on technically challenging and expensive approaches, researchers from Khaled Salama’s lab, in collaboration with others, developed a simple and scalable method that converts polymer or carbon precursor films into graphene electrodes using a laser beam. This mask-free method produces uniform, three-dimensional multilayered electrodes that combine high porosity and surface area, necessary for next-generation electrochemical sensor and biosensor platforms.
Salama’s team and collaborators from the Hassan II University of Casablanca, Morocco, incorporated laser-derived graphene (LDG) electrodes in sensing platforms for major sources of antioxidants called phenolic compounds and related electroactive biomolecules².
All tested compounds showed higher electrocatalytic activity on the graphene-based platforms than on conventional systems using carbon electrodes.
“The graphene-based platforms showed excellent performance for detecting paracetamol, a common drug,” says Abdellatif Ait Lahcen, a postdoc from Salama’s Lab. They also distinguished paracetamol in a commercially available tablet that combines the drug with the antioxidant ascorbic acid, which often produces interferences in typical electrochemical analyses.
Read the full article




