© 2021 KAUST; Gustavo Ramirez Calderon.
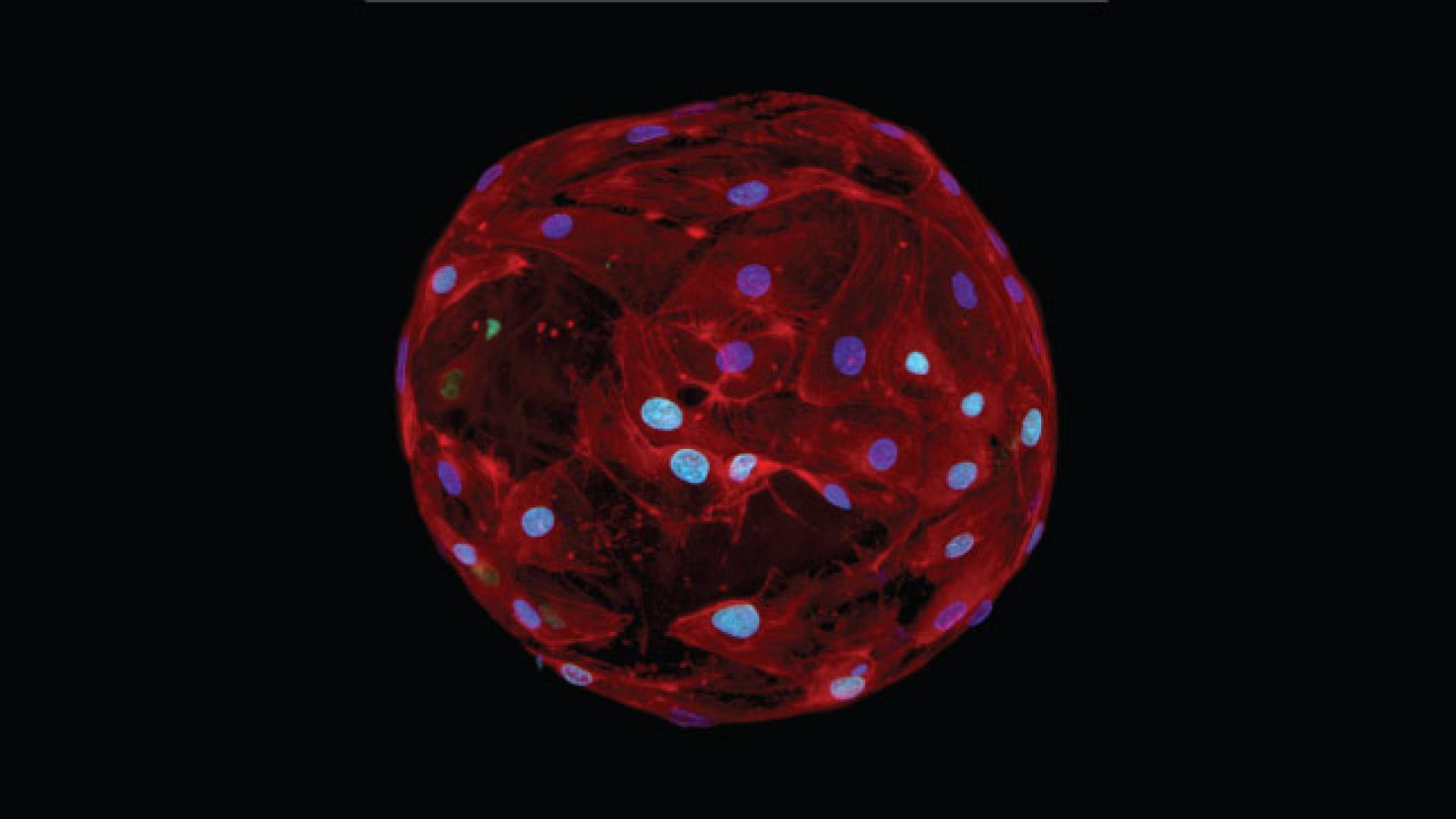
Micrometer-sized gel drops can provide the extracellular architecture needed for cells to grow and proliferate. The cell-carrying gels, made of self-assembling ultrashort peptides that form supportive nanofiber networks, might be injected into ischemic tissue in need of revival with new blood vessels.
“Our microgels are unique because they are made of only four amino acids, which is the shortest self-assembling peptide used to fabricate microgels so far,” says KAUST bioengineer Charlotte Hauser, who led the study. “This ultrashort structure reduces the cost and time for peptide synthesis.”
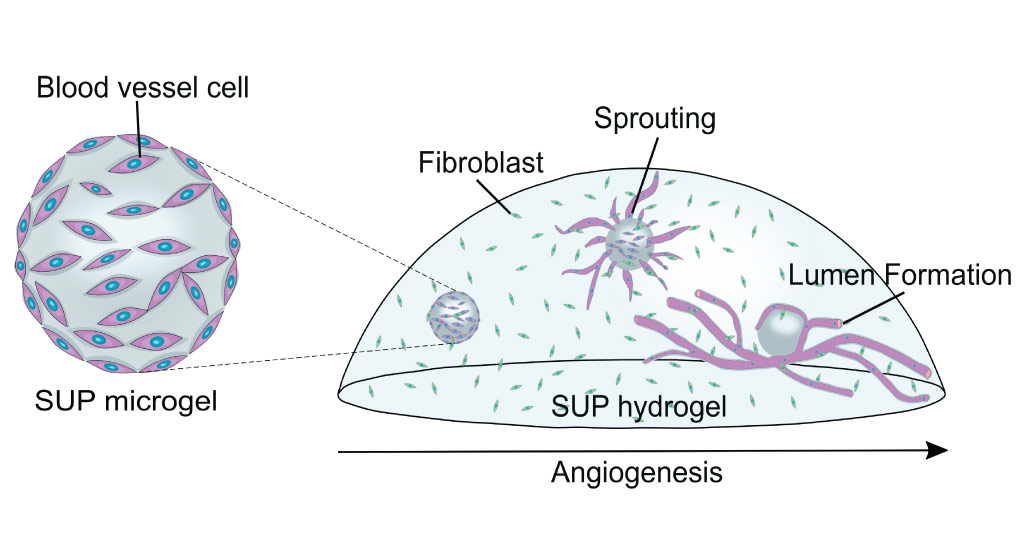
Scientists have been experimenting with various approaches for making human-like tissues that can be used for regenerative therapies. Self-assembling ultrashort peptides have an advantage over other materials because they can come together to form an architecture similar to that which supports cells in living tissue. They can also be made from chemically synthesized peptides that do not cause immune rejection by the body and are easily modified and upscaled for mass production.
Hauser and her team had been investigating the fabrication of microgels using self-assembling ultrashort peptides made of three and six amino acids. But they were struggling to optimize the gelling process that encourages the peptide networks to form into suitably shaped and sized droplets.
So they experimented with peptides made of four amino acids. Then most promising peptide was made by linking together the amino acids isoleucine, valine, phenylalanine and lysine, followed by adding an acetyl group to one end and an amide group to the other. Many of these peptides are put in an aqueous solution where they link together in a specific fashion that ultimately forms a fibrous network.
Read the full article