© 2021 Alshehri et al.
Tissue-engineering scaffolds built around ultrashort peptides provide a new platform for studying bone regeneration in the lab.
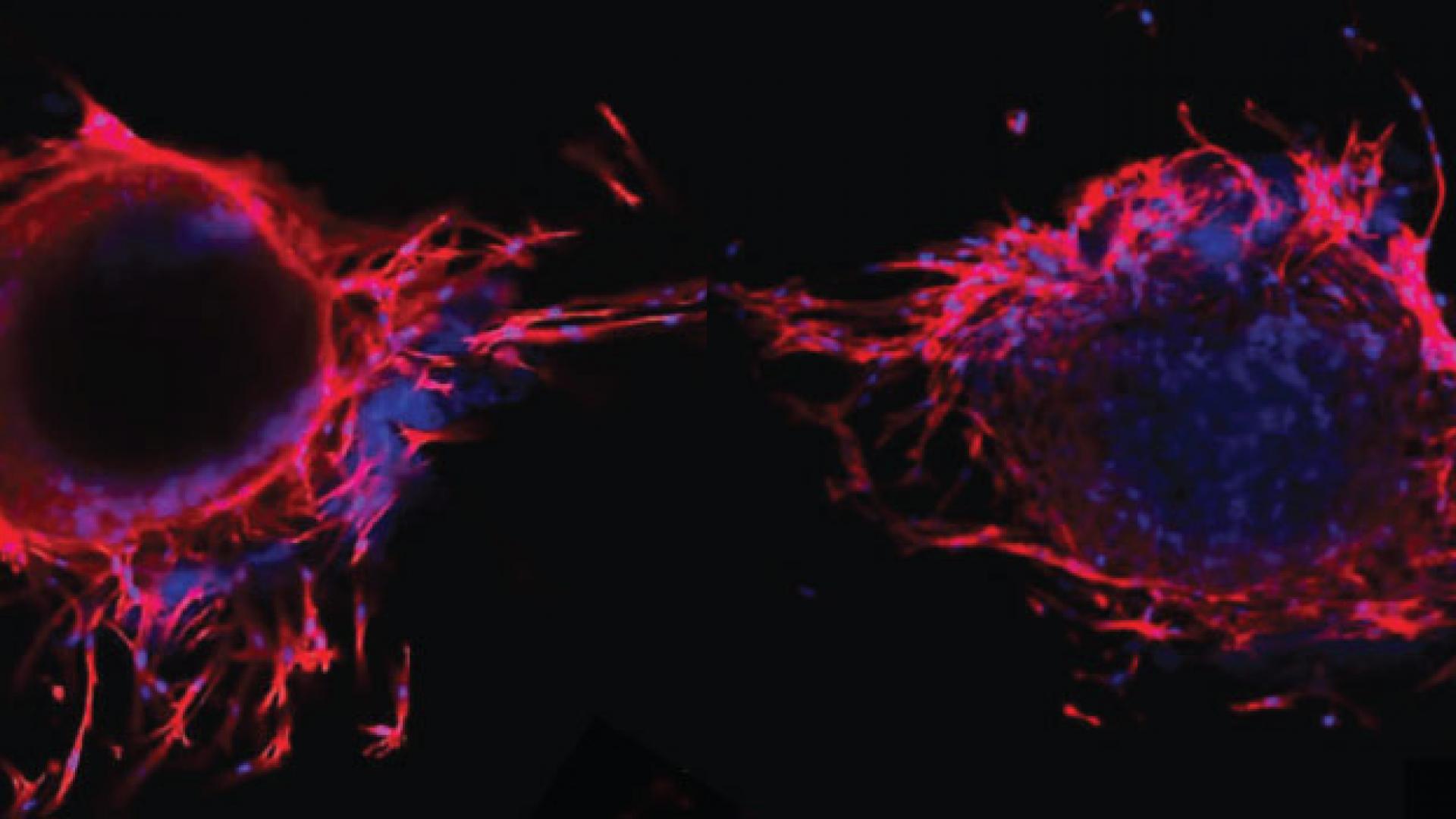
The peptides developed at KAUST self-assemble into a cartilage-like hydrogel that mimics the natural matrix that underpins bone formation in the body. Its physiologically relevant properties enable this cell-friendly biomaterial to support the growth and development of bone marrow precursor cells. It also enables tubular blood vessels to take shape, which is a critical part of bone health and repair.
“Our system is a simple, efficient and robust model that closely resembles the complex architecture of native bone tissue,” says Ph.D. student Salwa Alshehri. “Using these peptide-based hydrogels, we can now build 3D disease models for tissue engineering, biomedical research and drug testing.”
KAUST researchers, led by bioengineering professor Charlotte Hauser, had previously shown that their ultrashort peptides could be mixed with cells in the nozzle of a 3D printer to create a type of bioink that, once ejected, would instantly solidify into desired forms. But it was unclear if the synthetic material could sustain the full complexity of bone development, which includes the adhesion, spread and differentiation of bone-specific stem cells, along with the incursion of blood vessels needed for nutrient transfer and metabolic-waste removal.
Read the full article